Prevention of Post injection Endophthalmitis: Pearls in Real-world Scenario.
Before anything else, preparation is the key to success.”
– Alexander Graham Bell

Prof. (Dr.) Atul Kumar
Chief, Dr RP Centre for Ophthalmic Sciences,
AIIMS, New Delhi

Dr. Raghav Ravani
Senior Resident, Dr RP Centre for Ophthalmic Sciences,
AIIMS, New Delhi
With increase in life-expectancy and an epidemic of non-communicable disease throughout the world, especially in India, retinal conditions like diabetic macular edema (DME), Age related macular degeneration (AMD, especially neovascular AMD) and vein occlusions secondary to hypertension have become a major cause of visual impairment and visual loss. With a better understanding of the pathophysiology of these retinal conditions, it is now known that vascular endothelial growth factor (VEGF) plays a pivotal role in its pathogenesis. Anti-VEGF agents are the first-line drugs, which have shown efficacy not only in preventing vision loss, but also in improving visual acuity. Thus, with advent and use of newer anti-VEGF agent, there is a paradigm shift in the management of these retinal pathologies. The ability of the technique to achieve therapeutic levels locally with prolonged effective concentrations coupled with relative ease of performing the procedure, has made intravitreal injections the most common medical procedure (about twice the cataract surgery).
Though the incidence is low (0.016–0.026%), end ophthalmologist is a dreaded complication leading to severe ocular morbidity and vision loss. With dramatic increase in the number of injections performed annually in India and multiple patients undergoing the procedure in the same operating theater (OT) on the same day, there are confirmed reports of occurrence of cluster end ophthalmologist throughout the country. Since multiple patients undergo the procedure in one sitting, any breach in asepsis, cold-chain or contaminated drug increases the risk of cluster end ophthalmologists. This is, especially, true for intravitreal bevacizumab (Avastin®), available as 4-ml vial which is used for multiple patients at a time.

The intraocular use of multidose Avastin proves to be the cheapest and most cost-effective besides its high efficacy. However, it is “off-label” as it is not approved by the Food and Drug Administration, USA nor by the drug controller general of India for intravitreal use. This has led to a lot of debate and doubts regarding legal implications of the use of intravitreal Avastin®, procurement of the drug, precautions to be taken, and guidelines to be followed while using the vial for multiple patients. Recently, a cluster of 21 patients suffering from end ophthalmologist after being injected from a single vial of Avastin® on a single day was referred to our tertiary care center. A majority of these patients showed Stenotrophomonas maltophilia as the causative agent which was also isolated from the vial and is an emerging nosocomial infection causing endophthalmologist.
Thus, to avoid such dreaded complication like cluster end ophthalmologist, the procedure should be carried out diligently with proper pre-, intra-, and post-operative precautions and the best practice guidelines formulated and issued by VRSI, Dr. R.P. Centre (AIIMS, New Delhi), and AIOS, should be followed.
Drug procurement and storage:
- Since contaminated drugs or counterfeit drugs are an important cause of cluster end ophthalmologist, it is imperative that drugs should be purchased from authorized Roche dealers with proper receipt.
- Batch number of each vial should be noted in a register before opening the vial and the records should be maintained which might help to track the the vial or the batch in case of unfortunate event of end ophthalmitis.
- Cold chain should be maintained at each stage (2°–8° C, never freeze the vial), especially at dealer’s storage facility, transport to the hospital and in the hospital with proper temperature log maintenance.
Option for multiple injections from one vial
It is advisable to avoid repeated puncturing of bevacizumab vial to prepare injections for patients even on same sitting, as this can lead to contamination of the drug. Following are the options for multiple injections from one vial.
(A) Ideally – Compounding pharmacy to prepare single-dose ampoules/ aliquots should be practiced in sterile laboratories with Class 10 facility under laminar flow hood [4] as is practiced at ocular pharmacology department of our centre by a specially designed protocol (Fig. 1).
(B) Prepare multiple syringes by single puncture of vial under the laminar hood. Store the syringes
in sterile container at the proper temperature. Such syringes may be stored with minimal degradation of anti-VEGF activity. Send two such syringes for culture. If culture negative, use the syringes for injection. The stored syringes should be discarded after 2 weeks.
(C) In case facility for above two not available – Pool up to seven patients on the day of injection. Prepare seven aliquots of around 0.2 ml per syringe (one syringe for one patient) inside the OT
by single puncture of the vial after proper scrubbing and using aseptic technique. Re-cap the syringes with fresh sterile needles. Keep these syringes on a sterile surface. Only use these for the patients in the same session.
Discard the vial – It is NOT to be reused or repunctured.
Pre-operative Precautions:
- A written informed consent explaining the procedure and the risks involved. Off-label use to be included in consent and explained to patient
- Thorough preoperative screening and control of risk factors like localized adnexal infection or systemic condition are mandatory
- Each patient to be given clean OT gown, protective cap, and booties before entering the preoperative holding area/OT
- In the preoperative holding area/ or on table, the periocular skin should be cleaned with povidoneiodine 10% solution
- Surgical/procedural time-out to verify patient’s name, intravitreal agent, and laterality should be practiced before injection in each patient
- Bilateral injections are not recommended. Injection in the other eye should be spaced at least one to 2 weeks apart
- Prophylactic topical antibiotics: There is a lack of evidence to support pre-, peri-, or postinjection topical antibiotics. In fact, one of the studies showed a trend toward higher incidence. However, a short course of post-procedure prophylactic antibiotic may be used on surgeon’s personal experience and discretion.
Intraoperative Precautions:
- Location: The procedure should be performed in an OT setting, and not in office setting.
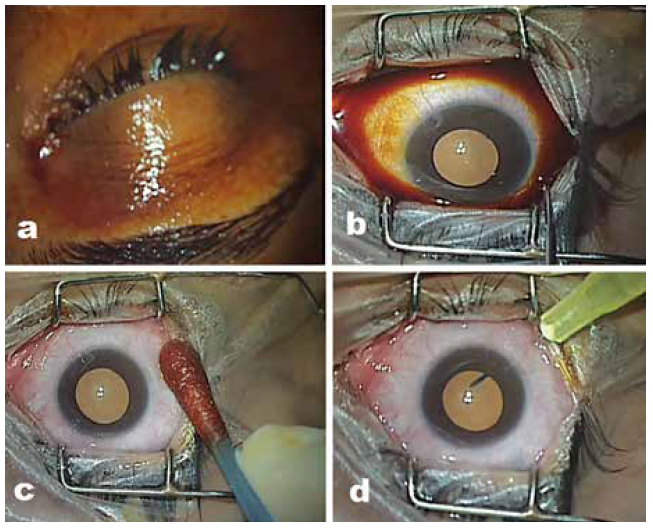
- Cleaning and draping: Use 10% povidone-iodine to clean skin and ocular adnexa (Fig. 2a), 5% povidone-iodine for instillation into cul-de-sac with contact time of at least 3 min (Fig. 2b). The surgical area should be draped using sterile linen and a separate plastic eye drape for each patient to isolate the field
- A speculum should be used to prevent contact of the eyelashes and eyelid margins with injection site and the needle
- Topical anesthetic drops should be preferred over anesthetic gel as the latter may interfere with povidone-iodine contact with the conjunctiva/injection site
- Reapply povidone-iodine after anesthetic drop use. Before injection, povidone-iodine (5%) should be the last agent applied to the intended injection site
- The surgeon/staff/patient should minimize speaking on table during preparation or during the injection procedure to minimize the spread of aerosolized droplets containing oral contaminants
- Conjunctival displacement and hemisphere of injection have no effect on the risk of infection
- Routine anterior chamber paracentesis is not recommended.
Postoperative Precautions:
- Postoperative antibiotics: Although, studies have not found any benefit of antibiotic prophylaxis, in country like India with poor ocular hygiene, post-injection topical antibiotics may be considered on surgeon’s discretion.
- Since postinjection endophthalmitis ranges from within 24 hrs. to up to 26 days post injection, with average
of 4 days, it is imperative to plan the first follow up visit early to look for earliest sign of end ophthalmologist and monitor intraocular pressure on follow up. - All patient should be give a discharge card mentioning procedure performed, injection details, post-operative instructions and medications, symptoms of infections (pain, redness, dimness of vision, swelling, discharge, etc.) and a 24 hour emergency contact information.
Numerous trials performed worldwide (CATT trial, IVAN trial, GEFAL, MANTA) on thousands of patients have shown intravitreal Bevacizumab (Avastin ®) to be noninferior to Lucentis (Ranibizumab®) in terms of efficacy and safety. Bevacizumab, on the other hand has the advantage of reducing the cost of therapy especially in our country where population’s access to resources is limited. Strict asepsis and proper technique goes a long way in providing desired outcomes and preventing dreadful complications like endophthalmitis.
References:
- McCannel CA, Flynn HW, Cunningham ET., Jr Updated Guidelines for Intravitreal Injection. Rev Ophthalmol.
- Dossarps D, Bron AM, Koehrer P, Aho-Glélé LS, Creuzot-Garcher C FRCR net (FRenCh Retina specialists net) Endophthalmitis after intravitreal injections: Incidence, presentation, management, and visual outcome. Am J Ophthalmol
- Results of the Endophthalmitis Vitrectomy Study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Endophthalmitis Vitrectomy Study Group. Arch Ophthalmol
- Kumar A, Ravani R. Using intravitreal bevacizumab (Avastin®) – Indian scenario. Indian J Ophthalmol.
- Bakri SJ, Snyder MR, Pulido JS, McCannel CA, Weiss WT, Singh RJ. Six-month stability of bevacizumab (Avastin) binding
to vascular endothelial growth factor after withdrawal into a syringe and refrigeration or freezing. Retina - Storey P, Dollin M, Pitcher J, Reddy S, Vojtko J, Vander J, et al. The role of topical antibiotic prophylaxis to prevent end ophthalmologist after intravitreal injection. Ophthalmology
- Chen E, Lin MY, Cox J, Brown DM. Endophthalmitis after intravitreal injection: The importance of viridans streptococci. Retina.
- Garg SJ, Dollin M, Hsu J, Storey P, Vander JF. Effect of a strict ‘No- Talking’ policy during intravitreal injection on post-injection end ophthalmologist. Ophthalmic Surg Lasers Imaging Retina
- Shah CP, Garg SJ, Vander JF, Brown GC, Kaiser RS, Haller JA Post-Injection Endophthalmitis (PIE) Study Team. Outcomes and risk factors associated with end ophthalmologist after intravitreal injection of anti-vascular endothelial growth factor agents.