“A joy that’s shared is a joy made double.”

Gautam Singh Parmar
Consultant & Head
Sadguru Netra Chikitsalaya
Chitrakoot, MP
Introduction
Hemi-Descemet membrane endothelial keratoplasty (hemi-DMEK) is a latest modification of conventional Descemet membrane endothelial kerato-plasty (DMEK). It differs from the conventional DMEK only in the graft shape because
instead of a circular trephined DMEK graft, hemi-DMEK uses full length (11-12mm), untrephined, semicircular (“half-moon” shaped) graft. The other half is transplanted to another recipient. Thus one donor cornea is transplanted into two recipients, thereby potentially doubling the availability of endothelial tissue (figure 1). This, to some extent, compen-sates for the scarcity of donor corneal tissue which is prevalent worldwide1, especially in a developing nation like India2.
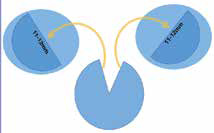
Figure 1: Endothelial tissue from single
donor, used for two recipients.
The concept of hemi-DMEK was introduced by Lie et al3. The authors men-tioned that preparation of two hemi-DMEK grafts is technically feasible, and the grafts can be stored in organ culture media like standard circular DMEK grafts.
Indications
Both DMEK and hemi-DMEK, in comparison to previous techniques, needs relatively clearer cornea to facilitate manipulation of donor endothelial tissue in anterior chamber of recipient which is crucial for proper orientation of tissue. Eyes with Fuch’s endothelial corneal dystrophy (FED) are the most suitable candidates for hemi-DMEK as these corneas have clearer and thinner stroma as well as pristine Descemet membrane (DM) which allow its easy stripping during surgery. The indications can be further extended in future to endothelial failure of any origin with clear stroma and thinner pachymetry.
Technique
As a modification of standard DMEK, hemi-DMEK is basically similar to DMEK. The technique as described by Lie JT et al2 is as follows:
The corneoscleral rim is mounted endothelial side up, over a holder with a suction cup. The DM is loosened 360 degrees from periphery towards center. The whole corneoscleral rim is bisected in two halves by 10 number surgical knife. Both halves are stained with trypan blue 0.06%. After removal of rem-nants of trabecular meshwork, the DM is stripped completely along the cut edge to make it free, from both halves, thus two semicircular, full length do-nor endothelial tissue are obtained. These grafts roll spontaneously with endothelium outside after immersion in saline. The long axis of roll is either per-pendicular to the long sharp edge or oblique to it, resulting in a hemi-DMEK graft shorter in length compared to a standard DMEK graft.
Graft insertion and placement:
Graft insertion technique in hemi- DMEK is similar to standard DMEK.A standard 8-9 mm descemetorhexis is made. Stained, ‘double-roll’ graft is injected as in standard DMEK, into recipient’s anterior chamber, with the en-dothelium facing down, towards the iris and donor DM facing up, towards stroma. Using indirect manipulation with the help of two canulae and with air and balanced salt solution, the graft is carefully unrolled keeping proper orientation. Graft is positioned in such a way that the longer diameter of the tissue lies across the longer, horizontal meridian of the eye. After complete un-rolling, an air bubble is injected beneath the graft to create air tamponade for attachment of graft. Air is left in anterior chamber for 60–90 minutes, after which a partial air-fluid exchange is done, leaving 50 percent air in anterior chamber at the end of operation. Unlike standard DMEK, stamping to facili-tate proper orientation of donor lenticule in eye, was not originally described for hemi- DMEK. It is done by observing DM roll with endothelium facing outward.
Outcomes
Visual outcome: Visual outcome in hemi-DMEK is comparable to conventional DMEK5-9. Though the visual recovery is slower as mentioned by Birbal et al5, entire corneal clearance after hemi- DMEK may be a bit slower than after conventional DMEK because of the bare areas of recipient stroma resulting from the mismatch of the circular descemetorhexis and the semicircular hemi-DMEK graft.
Donor endothelial cell density (ECD):
In hemi-DMEK, initial sharp decrease in ECD occurred within the first 3-6 months, which is higher than standard DMEK (34% vs 65%)5-9. After that yearly decrease in ECD is comparable to standard DMEK. This may be ex-plained by different patterns of endothelial cell redistribution and migration after hemi-DMEK (Figure 2) compared to conventional DMEK. In hemi-DMEK because of larger stromal bare areas of the recipient, initial migration and
redistribution of endothelial cells is faster than standard DMEK. Corneal pachymetry:
Corneal pachymetry shows significant reduction after hemi- DMEK5-9, as noticed in all patients. Rate of decrease in thickness is slower than conventional DMEK because the area of bare stroma adjacent to hemi-DMEK graft remain edematous for longer duration and complete clearance of stroma is achieved by 6 months9.
Complications
Graft detachment: The main early complication is graft detachment, for which rebubbling is required. A possible explanation for the higher detachment rate after hemi-DMEK than after conventional DMEK might be a “learning curve” of this modified technique5. One more reason for detachment of graft in hemi-DMEK is overlapping peripheral corners which overlap peripheral rim of intact DM because of poor adhesion between DM endothelium interface in contrast to central area of firm adhesion between DM and stromal interface (figure 2). Tourtas et al4 discussed in their study that overlapping zones seem to affect adhesion properties of the graft, because all the patients with graft detachment required rebubbling. Therefore, special care should be taken to position the graft at the area of bare stroma and avoid overlapping zones.
Allograft rejection:
The endothelial graft in hemi-DMEK remain in proximity to limbus. Despite that, no higher chance of graft rejection is noticed in hemi-DMEK as compared to standard DMEK. Conclusion Hemi-DMEK is technically feasible and offer comparable outcomes and complications as conventional DMEK. It potentially doubles the availability of suitable endothelial donor tissue. It also helps the corneal surgeon to utilises the accidentally torn donor tissue during graft preparation for conventional DMEK and hence avoid loss of a valuable graft. Hemi-DMEK may become the technique of choice for endothelial keratoplasty in future.
References
1. Heindl LM, Riss S, Bachmann BO, et al. Split cornea transplantation for 2 recipients: a new strategy to reduce corneal tissue cost and shortage. Ophthalmology. 2011; 118:294–301.
2. Vajpayee RB, Sharma N, Jhanji V, et al. One donor cornea for 3 recipients: a new concept for corneal transplantation surgery. Arch Ophthalmol 2007; 125:552–4.
3. Lie JT, Lam FC, Groeneveld-van Beek EA, et al. Graft preparation for hemi-Descemet membrane endothelial keratoplasty (hemi- DMEK). Br J Ophthalmol. 2016; 100:420–424.
4. Tourtas T, Heindl LM, Kopsachilis N, et al. Use of accidentally torn Descemet membrane to successfully complete
Descemet membrane endothelial keratoplasty. Cornea. 2013; 32:1418–1422.
5. Rénuka S. Birbal, Shugi Hsien, Vasiliki Zygoura et al, Outcomes of Hemi-Descemet Membrane Endothelial Keratoplasty for Fuchs Endothelial Corneal Dystrophy,Cornea, Volume 0, Number 0, Month 2018
6. Gerber-Hollbach N, Parker J, Baydoun L, et al. Preliminary outcome of hemi-Descemet membrane endothelial keratoplasty for Fuchs endothelial dystrophy. Br J Ophthalmol. 2016; 100:1564–1568.
7. Müller TM, Baydoun L, Melles GR. 3-Year update on the first case series of hemi-Descemet membrane endothelial
keratoplasty. Graefes Arch Clin Exp Ophthalmol. 2017; 255:213–215.
8. Lam FC, Baydoun L, Dirisamer M,Lie J, Dapena I, Melles GR (2014) Hemi-Descemet mem-brane endothelial keratoplasty transplantation. JAMA Ophthalmol 132:1469–1473
9. Lam FC, Baydoun L, Satué M, et al. One year outcome of hemi-Descemet membrane endotheli-al keratoplasty. Graefes Arch ClinExp Ophthalmol. 2015; 253:1955–1958